How Do Scientists Measure the Strength of Acids and Bases
Although there may be many types of ions in a solution pH focuses on concentrations of hydrogen ions H and hydroxide ions OH -. One of the earliest tests to determine acids from bases was the litmus test.

Acids And Bases Basic Introduction Chemistry Youtube
An indicators color depends on the concentration of _____ ions in the solution being tested.

. The scale measures values from 0 all the way up to 14. Distilled water is 7 right in the middle. What is an acid.
What did you already know about acids and bases before you began the webquest. On most pH meters the pH of the solution is displayed on a digital readout. Since electrolytes are created as ions are freed into solution there is a relationship between the strength of an acid a base and the electrolyte it produces.
The acidity of a solution is a measure of the concentration of the hydrogen ions in the solution. The base dissociation constant K b is a measure of basicitythe bases general strength. Describe the physical properties such as taste and feel of acids and bases.
A chemical patch turned red for acids blue for bases. Describe the physical properties such as taste and feel of acids and bases. An acid produces hydrogen ions in aqueous solution.
What is a base. How do scientists measure the strength of acids and bases. The acid and base dissociation constant is the measure of the strength of the acids and bases.
Describe the physical properties such as taste and feel of acids and bases. Up to 24 cash back pH meters can also test the strength of an acid or a base. To measure the strength of an acid you need to measure the amount of dissociation.
Acids and Bases Introduction. Learn about how the strength of acids and bases are determined in this videotranscript_____not all acids and bases are the same. Water can even react with itself to form acids and.
This scale ranges from _____ to _____. The acidity of a solution is a measure of the concentration of the hydrogen ions in the solution. Both an acid and a base depending on how you look at it.
The higher the dissociation constant the stronger the acid or base. The alkalinity of a solution is a measure of the concentration of the hydroxide ions in the solution. ACIDS AND BASES WEBQUEST Part 1.
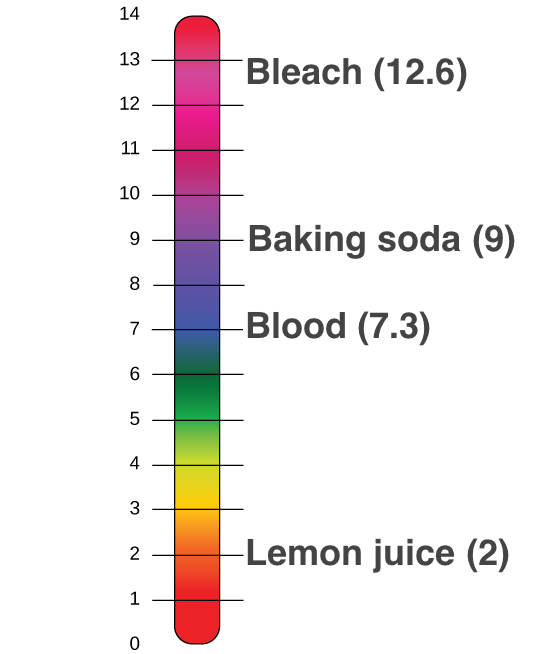
The strength of acids and bases is most often indicated by the concentration of hydrogen ions in the solution. Next we will take a look at some examples of acids and bases. The pH scale is the most common and trusted way to measure how acidic or basic a substance is.
To accomplish that you can use the pH as one of three measurements. The base dissociation constant can be expressed as follows. Up to 24 cash back THE STRENGTH OF ACIDS AND ALKALIS.
What determines the strength of acids and bases. How do scientists measure the strength of acids and bases what is the scale. How do scientists measure the strength of acids and bases.
Describe the physical properties such as taste and feel of acids and bases. How can we measure the strength of acids and alkalis. They use a pH meter.
____ of a solution refers to how completely an acid or base forms ions in solution. A base produces hydroxide ions in aqueous solution. Properties of Acids Neutral Properties of.
Another way to check if a substance is acidic or basic is to use litmus paper. Anything with a pH below 7 is acidic. This is called the pH.
Strength In solution strong acids and bases ionize ____ but weak acids and bases ionize only ____. To identify acids from bases and the relative strength of each chemists tend to use a pH scale. - Answers They use a pH meter.
This makes pH meters the most accurate way to measure pH. _____ _____ Part 4. The alkalinity of a solution is a measure of the concentration of the hydroxide ions in the solution.
How do scientists measure the strength of acids and bases. It is related to the acid dissociation constant K a by the simple relationship pK a pK b 14 where pK b and pK a are the negative logarithms of K b and K a respectively. A pH meter measures hydrogen ions in the solution tested.
Lower the more acidic Any value more than 7 is basicthe higher the more basic 7 is. A pH meter is the most accurate way to measure the pH of a solution. The first measurement is the mass convert to moles of the acid you dissolved.
How do you neutralize an acid or a base. Anything with a pH above 7 is basic. If the number of hydrogens has decreased that substance is the acid donates hydrogen ions.
How do scientists measure the strength of acids and bases. Guest SHOW ANSWER each column of the table is called a group. Scientists use something called the pH scale to measure how acidic or basic a liquid is.
Scientists measure the strength of acids and bases by using the ph scale. A base produces hydroxide ions in aqueous solution. To determine whether a substance is an acid or a base count the hydrogens on each substance before and after the reaction.
How do scientists measure the strength of acids and bases. The second is the final volume of the solution after you have dissolved and thoroughly mixed the acid. An acid produces hydrogen ions in aqueous solution.
It can be considered an acid in some reactions and a base in others. A pH scale measure can vary from 0 to 14 where 0 is the most acidic and 14 is the most basic a substance can be. Go to the VisionLearning Website and learn more about the properties of acids and bases.
The scale is called pH scale any value below 7 is acidic answered. Scientists use what is known as a _____ scale to measure the strength of acids and bases. The ph scale is a scale that measures the acidity or basic a liquid is.
Seven is considered _____. If the number of hydrogens has increased that substance is the base accepts hydrogen ions. Go to the and read about acids and bases.

Shielding Electrons Slater S Rule Effective Nuclear Charge Effective Nuclear Charge Ionization Energy Chemistry Basics
Ph Acids And Bases Review Article Khan Academy

Chemistry Intermolecular Forces And Boiling Points Intermolecular Force Chemistry Notes Teaching Tips
No comments for "How Do Scientists Measure the Strength of Acids and Bases"
Post a Comment